Abstract

Background: ENKTL is a highly aggressive NHL with a higher incidence in Asia. L-asparaginase containing chemotherapy regimens are the most frequently administered in first-line treatment with moderate to severe toxicities. In 2020 ASH meeting, we reported Sintilimab(anti-PD-1 antibody) plus Chidamide(an oral subtype-selective HDACi) yielded effective antitumor activity, durable response with mild toxicity in patients with relapsed or refractory ENKTL(SCENT trial. Abstracts 644) for the first time. We then initiated this exploratory study to investigate the efficacy and safety of Sintilimab plus Chidamide(SC) for patients with newly diagnosed ENKTL.
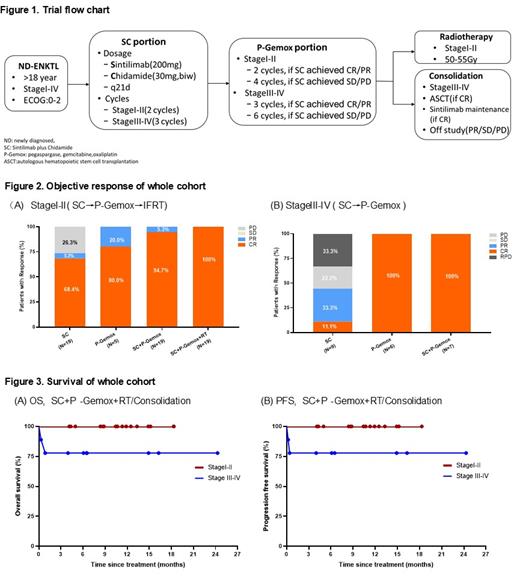
Methods: This trial enrolled eligible patients with newly diagnosed ENKTL(ND-ENKTL); ECOG score≤ 2; at least one measurable or evaluable lesion. Trial flow chart was showed in Figure 1. All patients received 2-3 cycles of Sintilimab (200mg) plus Chidamide (30mg, twice a week). For patients with early stage, 2 cycles of SC were given, subsequent 2-4 cycles of P-Gemox administered and followed by involved field radiotherapy (IFRT). For advanced stage, patients were treated with 3 cycles of SC and 3-6 cycles of P-Gemox. The primary study endpoints are the ORR of SC and end of treatment assessed according to the lymphoma response to immunomodulatory therapy criteria (LYRIC). Key secondary endpoints included DOR, PFS, OS and safety. Adverse events (AEs) were defined according to CTCAE 5.0. Pretreatment FFPE tumor and blood samples were obtained. Samples were analyzed by capture-based NGS targeting lymphoma relevant genes.
Results: From July 2019 to April 2021, 30 eligible patients were enrolled from Sun Yat-sen University Cancer Center. The median age was 49 (range, 21-81 )years, 2(6.7%) patients with ECOG score ≥2, 12(40%) patients with stage III-IV, 11(36.7%) patients with PINK-E score≥3. Nineteen stageI-II and 9 stage III-IV patients were evaluated for efficacy. For stage I-II, 14(73.7%) patients achieved response, including 13(68.4%) CR patients and 1 (5.3%) PR with SC. After 2 cycles of SC, 9(69.2%) CR patients chosen to continue SC treatment. Five (26.3%) PR patients accepted 2 cycles of P-GemOx, 4(80%) patients got CR. All patients obtained CR (100%) after IFRT(Fig.2A). For stage III-IV, 4(44.4%) patients achieved response (1 got CR), 3(33.3%) patients experienced rapid progression disease (RPD) in SC portion. The CR patient chosen to continue SC treatment. Six (66.7%) patients entered P-Gemox portion including 1 RPD patient, all (100%) patient got CR. At last follow-up, ORR was 100% (7/7) with 100% CR(Fig.2B). The median follow-up time was 8.8 (0.3-24.3) months. The estimated 1-year PFS and OS rate were all 92.9±0.5%( Fig.3 A-B). 2 RPD patients died within one month. Circulating EBV-DNA clearance after SC significantly correlated with superior outcome. Dynamics ctDNA assay is in progress, data in detail will be presented at the ASH conference. Twenty-nine(96.7%) patients reported treatment-related adverse effects (TRAEs). The most common TRAEs (≥10%) were neutropenia (43.3%), thrombocytopenia (36.7%), elevated transaminase (33.3%), and anemia (23.3%). The most common grade 3 TRAEs(≥10%) was neutropenia (13.3%), elevated transaminase (10.0%), no grade 4 TRAEs. There were 4(13.3%) cases of immune-related AEs, including 3 cases of hypothyroidism and 1 case of hyperthyroidism.
Conclusion: Sintilimab plus Chidamide yielded effective antitumor activity and manageable toxicities in newly diagnosed ENKTL for the first time. It may be a promising chemo-free induction therapeutic portion for this population, especially for early-stage patients. Further investigation is needed.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal